Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Ouchi, Kazuki; Komatsu, Atsushi; Takao, Koichiro*; Kitatsuji, Yoshihiro; Watanabe, Masayuki
Chemistry Letters, 50(6), p.1169 - 1172, 2021/06
Times Cited Count:1 Percentile:6.77(Chemistry, Multidisciplinary)The electrochemical behavior of uranium (IV) tetrachloride in ionic liquid-DMF mixture was studied for first time in order to build a redox flow battery (RFB) using U as an electrode active material. We found a quasi-reversible U /U
/U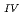 couple that could be applied to the anode reaction of the RFB.
couple that could be applied to the anode reaction of the RFB.
JAERI-M 5878, 124 Pages, 1974/10
no abstracts in English
Ouchi, Kazuki; Komatsu, Atsushi; Takao, Koichiro*; Kitatsuji, Yoshihiro; Watanabe, Masayuki
no journal, ,
The electrode reaction of uranium (IV) tetrachloride in an ionic liquid-DMF mixture was studied in order to build a redox flow battery (RFB) using U as an electrode active material. We found the quasi-reversible U(III)/U(IV) reaction that the formal potential is almost constant at -1.559  0.003 V. This reaction could be applied to the negative electrode reaction of the RFB.
0.003 V. This reaction could be applied to the negative electrode reaction of the RFB.